Recent Announcements
PhotoPharmics Closes Oversubscribed $6 Million Series B Extension to Advance FDA Breakthrough Parkinson’s Device
Original Press Release Funding supports key milestones as the Pivotal, Phase 3 trial surpasses 200...
PhotoPharmics Reaches 200 Participants in Landmark Light for PD Clinical Trial
Two-thirds enrolled in one of the largest remote Parkinson’s trials—redefining access and...
PhotoPharmics Announces Leadership Transition in Clinical & Scientific Advisory Board
PhotoPharmics, a pioneer in specialized phototherapy for neurodegenerative diseases, today...
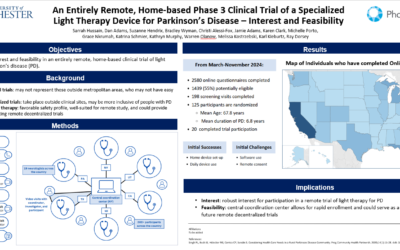
PhotoPharmics to Present Patient Interest Findings from Groundbreaking Remote Parkinson’s Trial at PSG Annual Meeting
Presentation to Highlight Feasibility and Interest in Decentralized Home-Based Phase 3 Trial Salt...
Featured Article

10 Ways to Manage Your Parkinson’s Symptoms
Introduction Living with Parkinson's disease presents a unique set of challenges, both physically and emotionally. While medication plays a crucial role in managing the condition, a holistic approach that incorporates various lifestyle adjustments and self-care...
10 Ways to Manage Your Parkinson’s Symptoms
By actively incorporating regular exercise, a balanced diet, mental health strategies, quality sleep habits, social engagement, mindfulness, effective medication management, environmental adaptations, cognitive stimulation, and exploring complementary therapies, individuals living with Parkinson’s can take a proactive role in managing their symptoms, maintaining their independence, and enhancing their overall quality of life.
Parkinson’s disease is often thought of as a movement disorder—but in reality, many symptoms appear long before noticeable tremors or stiffness begin.
These early signs are diverse, affecting not only how a person moves but also how their body functions on a day-to-day level.
Understanding the early symptoms is essential for earlier diagnosis, timely intervention, and better quality of life.
🔄 Movement Symptoms (The “motor” signs):
These are the symptoms most people associate with Parkinson’s:
🔹 Tremor – often begins in one hand, especially at rest
🔹 Bradykinesia – slowness in movement, making daily tasks harder
🔹 Muscle stiffness or rigidity – reduced flexibility, sometimes mistaken for arthritis
Unsteady posture or walking pattern – small, shuffling steps
🔹 Masked facial expression – reduced facial emotion or fixed appearance
🔹 Decreased blinking – noticeable dryness or staring appearance
🧠 Non-Movement Symptoms (Often show up first):
Many early signs are subtle and not related to movement at all:
🔹 Loss of smell (anosmia) – one of the earliest clues
🔹 Constipation – slowing of the digestive system
🔹 Drooling – due to reduced automatic swallowing
🔹 Sleep disturbances – vivid dreams, restlessness, or acting out in sleep
🔹 Difficulty swallowing – may start gradually and worsen over time
These symptoms don’t appear all at once, and many overlap with normal aging or other conditions.
But spotting them early can help people seek medical advice and get on the right path sooner.
💬 Which of these early symptoms do you think is least recognized? Let`s talk below.
#EarlyDetection #Neuroscience #MotorSymptoms #NonMotorSymptoms #ParkinsonsSupport #KnowTheSigns #LightForPD #parkinsonsdisease #parkinsons #parkinsonsawareness #parkinsondisease #parkinsonsdiseaseawareness #parkinsonawareness #caregiver #goodnews
Parkinson’s disease is a complex, progressive neurological disorder, but the cause isn’t always clear.
In most cases, it’s not due to a single trigger, but rather a combination of genetic, environmental, and biological factors working together.
Here’s what we currently understand:
1. Genetic Causes (10–15% of cases)
Some people inherit Parkinson’s through changes in specific genes.
So far, researchers have identified several genetic mutations linked to the disease.
A few are associated with early-onset Parkinson’s (before age 50), while others may increase overall risk.
However, most people with Parkinson’s don’t have a clear family history—so genetics alone can’t explain the majority of cases.
2. Protein Misfolding & Lewy Body Buildup
In idiopathic (cause-unknown) Parkinson’s, scientists believe the problem starts with misfolded α-synuclein protein.
When this protein clumps together in brain cells, it forms what are called Lewy bodies.
These toxic buildups interfere with brain function and eventually lead to the death of dopamine-producing neurons—a key feature of Parkinson’s progression.
3. Environmental Factors
Long-term exposure to certain pesticides, heavy metals, or industrial chemicals may increase Parkinson’s risk.
In contrast, some factors—like smoking or caffeine—have been linked to slightly lower risk (though not recommended as prevention).
Repeated head injuries are also associated with increased risk, especially in athletes and military populations.
➡️ Bottom Line:
Parkinson’s isn’t caused by one thing.
It’s shaped by the interplay of genes, misfolded proteins, and environmental exposures, and research continues to uncover how they interact.
Looking to get more insight on Parkinson`s? Follow Photopharmics!
#Neurology #BrainHealth #Genetics #EnvironmentalHealth #ParkinsonsResearch #LightForPD #parkinsonsdisease #parkinsons #parkinsonsawareness #parkinsondisease #parkinsonsdiseaseawareness #parkinsonawareness #caregiver #goodnews
Parkinson’s isn’t just about tremors.
Long before any movement symptoms appear, many people with Parkinson’s experience non-motor symptoms—often subtle, often overlooked.
We’d love to hear from you:
Which of these early non-motor symptoms did you notice?
🔹 Loss of smell (anosmia)
🔹 Anxiety or mood changes
🔹 Constipation or digestive issues
🔹 Sleep disturbances (REM behavior disorder, vivid dreams, insomnia)
These symptoms can appear years before a Parkinson’s diagnosis and are crucial early warning signs.
Yet many people (and even clinicians) don’t always associate them with Parkinson’s—leading to delayed diagnosis and support.
🗣️ Your experience can help others feel seen and encourage more open conversations around early signs of PD.
Looking for new ways to manage your symptoms?
The Light for PD clinical trial is exploring an innovative, non-invasive light-based therapy designed to support people with Parkinson’s—particularly those affected by non-motor symptoms like sleep disruption and fatigue.
It’s free to participate and could be a step toward better daily living with PD.
👇 Share your earliest symptoms below.
And if you`re curious about the trial, visit lightforpd.com to learn more.
#NonMotorSymptoms #ClinicalTrials #SleepInParkinsons #PDsupport #LightForPD #parkinsonsdisease #parkinsons #parkinsonsawareness #parkinsondisease #parkinsonsdiseaseawareness #parkinsonawareness #caregiver #goodnews
Struggling with sleep because of Parkinson’s?
You’re not alone.
Hear from Jimmy Choi, Parkinson’s Advocate, who was diagnosed with Parkinson’s Disease at age 27 and is now a 5-Time American Ninja Warrior about what you can do to help those symptoms that affect your daily life aside from the motor symptoms.
Because better sleep means better energy, mood, and quality of life.
Learn how light therapy might support your sleep and help you reclaim your nights.
👉 Tap the link in bio or visit lightforpd.com to learn about a clinical trial focused on the non-motor symptoms of Parkinson’s.
#ParkinsonsDisease #ParkinsonsAwareness #BetterSleep #PDsupport #LightTherapy #parkinsons #parkinsonsexercise #parkinsonswarrior #parkinsonsresearch #parkinsonssupport #parkinsonsdiseaseawareness #parkinsonssucks #parkinsonsfoundation #parkinsonspower #worldparkinsonsday #parkinsonslookslikeme #parkinsonsjourney #parkinsonscare #healthandwellness #physicaltherapy #chronicpainwarrior #seniorcitizen #findacure
There’s no single test for Parkinson’s disease. Instead, doctors rely on careful observation, history, and sometimes imaging to arrive at a diagnosis. It’s more about ruling out what it isn’t — and seeing how your body responds over time.
Here’s how the process typically unfolds:
🔹 Neurological Exam
Movement specialists assess slowness (bradykinesia), tremor, rigidity, posture, and coordination. A diagnosis usually requires slowness plus either tremor or stiffness.
🔹 Medical History
Doctors ask about your symptom timeline, family background, medications, and past injuries to rule out lookalikes.
🔹 Medication Challenge
If your symptoms improve with levodopa, it supports a PD diagnosis. But it’s not always conclusive.
🔹 Imaging Tests
MRIs or DaTscans don’t diagnose PD, but they help rule out strokes, tumors, or similar disorders.
🔹 Genetic Testing
In early-onset or family-linked cases, gene testing may help identify inherited forms of PD.
🔹 Blood Tests
Not to diagnose PD directly, but to rule out other causes like thyroid issues or Wilson’s disease.
🔹 Long-Term Monitoring
Sometimes, the best way to confirm PD is to track symptoms over time and see how they evolve.
Parkinson’s is a slow-moving condition — and so is diagnosing it. If in doubt, get a second opinion or consult a movement disorder specialist.
#neurology #dopamine #earlydiagnosis #parkinsonsresearch #parkinsonssupport #parkinsonslife #LightForPD #parkinsonsdisease #parkinsons #parkinsonsawareness #parkinsondisease #parkinsonsdiseaseawareness #parkinsonawareness #caregiver #goodnews
Living with Parkinson’s isn’t just about medication—it’s about empowerment through self-care.
From staying active to eating well, prioritizing sleep, and maintaining mental wellness, small daily actions can have a big impact.
Here’s a quick glimpse of what the blog covers:
— The kind of exercise that helps improve balance, strength, and mood
— How a nutritious diet supports both the body and brain
— Practical tips to improve sleep quality and reduce fatigue
— Ways to nurture your emotional health and stay socially connected
— Insight into complementary therapies like yoga, massage, and tai chi
And much more—including how to adapt to your environment and stay mentally sharp
Whether you`re newly diagnosed or navigating the later stages, these ten strategies are tools to help you regain control and live fully.
📖 Ready to dive in?
Read the full blog here: https://photopharmics.com/10-ways-to-manage-your-parkinsons-symptoms-and-enhance-your-life/
#parkinsonssupport #parkinsonslife #parkinsonscommunity #selfcare #mentalhealth #parkinsonswellness #movementdisorder #neurodegenerative #caregiver #livingwithparkinsons #LightForPD #parkinsonsdisease #parkinsons #parkinsonsawareness #parkinsondisease #parkinsonsdiseaseawareness #parkinsonawareness #caregiver #goodnews
What we put on our plate matters — especially for those living with Parkinson’s disease.
While there’s no single “Parkinson’s diet,” certain foods may worsen symptoms or interfere with treatment.
🔹 Foods High in Saturated Fat
Research links diets rich in saturated fats to higher risk and faster progression of Parkinson’s. Try limiting foods like:
— Butter, lard, and cheese
— Fatty cuts of beef
— Palm oil and fried or heavily baked goods
While the ketogenic diet (which is high in fat) may help some, it’s not for everyone. Always consult your doctor before trying new plans.
🔹 Hard-to-Chew Foods
Over one-third of people with Parkinson’s experience difficulty swallowing. To avoid choking and ensure proper nutrition, opt for soft, easy-to-chew meals. A speech-language therapist can also offer safe eating strategies.
🔹 Processed Foods
Canned, fried foods, and sodas (yes, even the diet ones) are often linked with faster symptom progression and gut health issues. Better choices? Whole, unprocessed ingredients.
🧠 Better Diets for Parkinson’s?
Researchers recommend:
— MIND Diet (a blend of DASH and Mediterranean diets) for slowing progression
— Mediterranean Diet for cognitive health and reduced inflammation
— Keto Diet for possible motor improvement, though it carries risks like constipation and high cholesterol — especially in older adults
💡 Lifestyle Tips to Support Your Diet
— Drink plenty of water (at least 6–8 glasses/day)
— Get sunlight and fresh air for a natural Vitamin D boost
— Stay active — even light movement helps
— Talk to your doctor about safe supplements
A balanced, mindful approach to food and lifestyle can help ease symptoms and improve quality of life.
#parkinsonsdiet #parkinsonsfood #parkinsonshealth #parkinsonssupport #LightForPD #parkinsonsdisease #parkinsons #parkinsonsawareness #parkinsondisease #parkinsonsdiseaseawareness #parkinsonawareness #caregiver #goodnews
Parkinson’s doesn’t always start with a tremor.
The early signs can be subtle — and easily mistaken for normal aging or stress. However, paying close attention to these symptoms could help with earlier diagnosis and timely support.
Here are some early symptoms you shouldn’t ignore:
• Trouble sleeping or frequent disturbances at night
• Persistent fatigue that rest doesn’t fix
• A fading sense of smell — even your favorite foods may lose their aroma
• Handwriting that starts shrinking with each word
• Tremors or shaky movements, especially in the hands
• Slowness in movement — taking longer to do everyday tasks
• Muscle stiffness, inflexibility, or cramping that makes movement harder
• Feelings of anxiety or restlessness without a clear cause
• Unexplained sadness or emotional numbness that lasts weeks
• Bladder urgency or bowel irregularities
Each of these on their own may not mean Parkinson’s. But if you or someone you love is experiencing a few of them together, it’s worth having a conversation with a doctor.
🩺 Early detection doesn’t just lead to early treatment — it empowers individuals to make informed decisions, adapt their routines, and maintain a better quality of life.
✅ Don’t ignore the early signs. Talk to your healthcare provider and take proactive steps toward understanding your body better.
📌 Awareness is the first step in fighting back.
#earlydetectionsaveslives #neurology #brainhealth #tremorawareness #movementdisorders #caregiverlife #healthawareness #selfadvocacy #knowyoursymptoms #LightForPD #parkinsonsdisease #parkinsons #parkinsonsawareness #parkinsondisease #parkinsonsdiseaseawareness #parkinsonawareness #caregiver #goodnews
The Crucial Role of Dopamine in Parkinson’s Disease: What You Need to Know
Parkinson’s disease (PD) affects millions across the globe — and at its core lies one key player: dopamine.
This vital chemical messenger is responsible for coordinating smooth, controlled movements.
But in PD, the neurons that produce dopamine slowly begin to die off, particularly in an area of the brain known as the substantia nigra pars compacta.
👉 This gradual loss is what gives rise to the classic symptoms of PD — tremors, stiffness, and slow movement.
🔍 But what makes dopamine so important… and so dangerous?
The Dopamine Dilemma in Parkinson’s:
Dopaminergic neurons in the brain are highly specialized but also extremely vulnerable.
In PD, both dopamine levels and the neurons that produce it decline significantly.
Environmental toxins, genetic predispositions, and cellular stress all disrupt dopamine’s life cycle — from synthesis to breakdown.
Though essential, dopamine is highly reactive.
When it breaks down — naturally or through enzymatic reactions — it produces toxic byproducts:
— Reactive Oxygen Species (ROS): damage DNA and cell structures.
— Dopamine Quinones (DAQs): interfere with protein function.
— DOPAL: a highly reactive molecule linked to neuronal death.
These compounds build up over time, creating oxidative stress that slowly poisons the very cells that make dopamine.
❓ Why This Matter?
Understanding how dopamine metabolism goes wrong in PD is not just academic — it’s key to future therapies.
Scientists are actively working to:
✔️ Protect remaining dopamine-producing neurons
✔️ Develop treatments that neutralize toxic byproducts
✔️ Slow or stop the progression of PD altogether
This deeper insight into dopamine’s double-edged role offers hope for more targeted, effective therapies — and a better quality of life for those living with Parkinson’s.
#dopamine #dopamineinparkinsons #LightForPD #parkinsonsdisease #parkinsons #parkinsonsawareness #parkinsondisease #parkinsonsdiseaseawareness #parkinsonawareness #caregiver #goodnews
Struggling with sleep because of Parkinson’s? You’re not alone.
In this video, we break down why Parkinson’s affects your rest—and what you can do about it. 💤
Because better sleep means better energy, mood, and quality of life.
Learn how light therapy might support your sleep and help you reclaim your nights.
👉 Tap the link in bio or visit lightforpd.com to learn about a clinical trial focused on the non-motor symptoms of Parkinson’s.
#ParkinsonsDisease #ParkinsonsAwareness #BetterSleep #PDsupport #LightTherapy #LightForPD #CelesteTrial
Finding Strength Through Parkinson’s: Rich’s Story
“When I was diagnosed with young-onset Parkinson’s in 2015, my life felt like a scene from The Wizard of Oz—swept into a storm I never saw coming.”
For Rich, a successful TV producer, Parkinson’s arrived like an uninvited guest.
Tremors, stiffness, and uncertainty clouded his future. “I felt like the Tin Man—rusted, stuck, unsure of my next move.”
➡️ But instead of letting fear define him, Rich fought back.
It wasn’t easy. Failed treatments left him struggling, but he refused to give up. Deep brain stimulation (DBS) became his turning point, easing his symptoms and restoring his sense of self.
“Like the Tin Man getting his oil, I finally felt like I could move forward again.”
But Rich’s journey didn’t stop with his own healing.
He turned his experience into advocacy, collaborating with nonprofits, sharing his story with medical students, and producing meaningful projects.
📩 His message?
Parkinson’s may change your path, but it doesn’t have to take your purpose.
“The unwavering love of my girlfriend and daughter reminded me I am more than my diagnosis.
Gratitude became my armor, and I found strength in embracing life fully.”
Like Dorothy, Rich discovered that there’s no place like home—and no greater journey than rediscovering your heart.
This story was originally published on ParkinsonsFoundation.org.
Visit their website to read the full story!
#ParkinsonsJourney #Inspiration #DBS #HopeBeyondDiagnosis #FindingStrength #LightForPD #parkinsonsdisease #parkinsons #parkinsonsawareness #parkinsondisease #parkinsonsdiseaseawareness #caregiver #goodnews
Maintaining Dental Health in Parkinson’s: Why It Matters
Dental health isn’t just about a bright smile—it’s a crucial part of overall well-being, especially for people with Parkinson’s.
PD-related symptoms like tremors, muscle stiffness, and swallowing difficulties can make oral care challenging, increasing the risk of infections and complications.
❓ Why It’s Important:
Difficulty chewing due to missing teeth or weak muscles can impact nutrition and speech.
Swallowing issues may lead to choking or aspiration pneumonia.
Infected teeth and gums can spread bacteria, affecting overall health.
Tremors and rigidity can make brushing difficult, increasing the risk of cavities and gum disease.
Dry mouth from medication raises the likelihood of tooth decay.
❓ How to Maintain Dental Health with Parkinson’s:
✔ Use adaptive tools – electric toothbrushes and flossing aids can make oral care easier.
✔ Stay hydrated – sip water frequently or use dry-mouth products to prevent cavities.
✔ Routine dental visits – regular checkups help catch issues early.
✔ Modify your diet – soft, nutritious foods can make chewing easier while protecting your teeth.
✔ Communicate with your dentist – discuss any mobility or medication-related concerns.
Taking proactive steps can prevent complications and improve quality of life.
What has helped you maintain good oral health with Parkinson’s?
Share your tips in the comments below!
#ParkinsonsCare #DentalHealth #OralHygiene #LivingWellWithPD #SupportCommunity #LightForPD #parkinsonsdisease #parkinsons #parkinsonsawareness #parkinsondisease #parkinsonsdiseaseawareness #caregiver #goodnews
Parkinson’s can affect foot health, leading to stiffness, pain, and mobility issues.
Understanding these challenges and taking proactive steps can help improve comfort and movement.
➡️ Common Foot Issues & Management
1. Gait & Balance Problems
Parkinson’s often causes shuffling, shorter steps, and balance difficulties, increasing the risk of falls.
🔹 Try: Physiotherapy, supportive footwear, and gait training to improve walking patterns.
2. Stiffness & Flat-Footed Gait
Muscle rigidity and fallen arches can make movement difficult, leading to pain and fatigue.
🔹 Try: Wear well-fitted shoes with arch support, use orthotic insoles, and practice foot exercises.
3. Swelling (Oedema)
Reduced mobility can lead to fluid buildup, causing feet and ankles to feel heavy and tight.
🔹 Try: Elevating feet when sitting, ankle rotations to promote circulation, compression socks, and discussing medication adjustments if needed.
4. Dystonia & Toe Curling
Involuntary muscle contractions can lead to toe curling, inward-turning ankles, or foot drop, making walking painful and increasing fall risk.
🔹 Try: Toe splints, foot braces, stretching exercises, and consulting a specialist for medication adjustments.
Essential Foot Care Tips
Wear supportive, adjustable footwear to maintain stability and reduce strain.
Keep feet clean, dry, and moisturized (avoiding moisture between toes).
Perform daily foot and ankle exercises to improve flexibility and circulation.
Use orthotic insoles to relieve pressure and improve posture.
Avoid prolonged sitting—gentle movement can reduce stiffness and swelling.
Seek professional foot care from a podiatrist for custom solutions like insoles or therapy.
👣 Taking small steps to care for your feet can improve mobility, reduce pain, and help maintain independence.
If foot issues persist, consult a specialist for tailored solutions.
#ParkinsonsCare #FootHealth #StayActive #LightForPD #parkinsonsdisease #parkinsons #parkinsonsawareness #parkinsondisease #parkinsonsdiseaseawareness #caregiver #goodnews
❓ What Is One Thing Parkinson’s Disease Has Taught You?
"The biggest lessons come from the hardest battles. What has Parkinson’s taught you?"
Parkinson’s isn’t just a diagnosis—it’s a lifelong teacher.
It challenges the way you move, thinks, and feel, but it also reveals an inner strength you may never have realized before.
🔹 For some, it teaches patience—learning to navigate each day at a different pace. For others, it fosters resilience—the ability to adapt, push forward, and find new ways to keep going.
Many discover the power of community, recognizing that they are not alone in this journey.
And for some, it’s a lesson in gratitude—cherishing the small victories, the moments of joy, and the people who stand by their side.
No two experiences with Parkinson’s are the same, but the wisdom gained is invaluable.
➡️ Today, we invite you to share: What is one lesson Parkinson’s has taught you?
Your words might resonate with someone who needs encouragement today. Let’s learn from each other and build a stronger, more connected community.
Drop your thoughts in the comments. 💬👇
#ParkinsonsLessons #ShareYourStory #YouAreNotAlone #LightForPD #parkinsonsdisease #parkinsons #parkinsonsawareness #parkinsondisease #parkinsonsdiseaseawareness #caregiver #goodnews
Hallucinations and delusions can be unsettling for people with Parkinson’s and their families.
While these symptoms can be challenging, there are effective ways to handle them with care and understanding. Here are five strategies to help:
🔹 Have Open Conversations With Your Doctor
It’s easy to dismiss or hide hallucinations out of fear or embarrassment, but being upfront with your doctor is essential.
These symptoms can be linked to medication side effects, infections, or other medical conditions.
Identifying the cause is the first step toward managing them effectively.
🔹 Reassess Medications With Your Healthcare Team
Some Parkinson’s medications may contribute to hallucinations or delusions, especially when doses are changed.
A doctor might adjust prescriptions, reduce dosages, or introduce new treatments to help balance symptom management without worsening motor function.
Always consult a medical professional before making any changes.
🔹 Stay Calm and Offer Comfort
It can be distressing to witness a loved one experience a hallucination.
Instead of arguing about what’s real, provide reassurance.
A soothing presence—whether through touch, words or simply staying close—can help ease their anxiety and confusion.
🔹 Redirect Attention With Gentle Distractions
If someone is struggling with a delusion, shifting focus to a different activity or environment may help.
Moving to another room, starting a conversation, or engaging in a simple task can provide relief without confrontation.
🔹 Make the Home Environment Safer
Good lighting can reduce the occurrence of visual hallucinations, especially at night.
Keeping the home well-lit and securing potentially dangerous objects like sharp tools or car keys can prevent harm if confusion escalates.
Hallucinations and delusions can be difficult, but with patience and the right strategies, they can be managed.
If these symptoms persist or worsen, seeking professional guidance is crucial.
💙 Have you or a loved one experienced Parkinson’s hallucinations? What strategies have worked for you? Share in the comments below.
#LightForPD #parkinsonsdisease #parkinsons #parkinsonsdiseaseawareness
Navigating Parkinson`s Disease (PD) as a person of color can bring unique challenges, but building strong relationships with your healthcare team is key.
It`s essential to find providers who truly see and hear you!
Many in our community treat that first visit as an important interview.
Here`s a reminder of how to foster a trusting partnership with your healthcare provider:
🔹Connect: Introduce yourself and your care partner, make eye contact, and look for that human connection.
Do they acknowledge you and take the time to understand who you are?
🔹Listen: Does your provider actively listen to your questions, goals, and challenges?
Mutual understanding is the foundation of good care.
🔹Prepare: Do your homework before appointments!
Come with questions and feedback, and don`t hesitate to communicate between visits if possible.
🔹Communicate: Engage in open dialogue, establish shared treatment goals, and look for empathy and understanding in your interactions.
📍 Be aware that racial bias and misperceptions can unfortunately occur in healthcare settings.
You are not alone if you`ve experienced:
➡️ Being told, "You seem angry all the time," when it could be facial masking, a symptom of PD.
➡️ Facing the misconception that "young Black men don`t get PD" – remember, PD affects all people.
➡️ Hearing "You don`t fit the textbook example" – PD presents differently in everyone.
If you don`t feel heard or respected or that your provider is working with you, it`s okay to seek another healthcare professional.
Your well-being and effective management of your PD journey depend on a trusting and reciprocal relationship.
You deserve a healthcare team that sees you, understands your unique experience, and works collaboratively towards your best possible life.
#ParkinsonsDisease #PDCommunity #HealthcareEquity #RacialBias #PatientAdvocacy #TrustYourCare #LightForPD #parkinsonsdisease #parkinsons #parkinsonsawareness #parkinsondisease #parkinsonsdiseaseawareness #parkinsonawareness #caregiver #goodnews
Living far from a loved one with Parkinson`s Disease (PD) can bring a sense of helplessness, but distance doesn`t equate to inaction.
You absolutely can provide meaningful support!
While primary, hands-on care might be limited, your role as a secondary caregiver from afar is crucial.
It starts with being informed. Stay well-versed in your loved one`s health, medications, and care team.
Keep important contact information and documents readily accessible.
Regular communication with both the person with PD and their local caregivers – whether family, aides, or facility staff – is vital to understanding evolving needs.
Think beyond physical presence. Long-distance caregiving involves:
🔹 Consistent Connection: Schedule weekly calls and utilize video chats to stay connected visually and emotionally. Don`t assume "no news is good news."
🔹 Financial Support: Tactfully inquire about financial needs. Offer specific help like paying for medications, groceries, or household bills.
🔹 Caregiver Support: Send surprise gifts monthly to the local caregiver – flowers, a meal out, or a pampering basket can provide a much-needed boost.
🔹 Regular Visits: Budget for trips to check in, offer respite to the primary caregiver, and even attend doctor`s appointments.
🔹 Respite Options: If you can`t provide in-person respite, explore and offer to fund respite stays in care facilities or invite your loved one for a visit.
Remember, avoid offering unsolicited advice as a substitute for hands-on help, and refrain from making promises you can`t keep.
Your role is to be a reliable source of support, information, and care from afar. Long-distance caregiving is real, and your efforts truly matter.
#ParkinsonsCaregiving #LongDistanceSupport #PDBelievers #CaregiverLove #DistanceDoesntMatter #LightForPD #parkinsonsdisease #parkinsons #parkinsonsawareness #parkinsondisease #parkinsonsdiseaseawareness #parkinsonawareness #caregiver #goodnews
Caring for a loved one can be deeply fulfilling—but it can also be overwhelming.
When stress builds up, care partner burnout can set in, leading to exhaustion, frustration, and even withdrawal.
The key to avoiding burnout is recognizing the signs early and taking steps to care for yourself, too.
Here are four essential ways to prevent and recover from care partner burnout:
1—Talk to Someone
Bottling up emotions can make stress worse. Talk to a trusted friend, family member, or therapist about what you’re going through.
Connecting with others who understand can lighten the load and provide valuable perspective.
2—Ask for Help
You don’t have to do everything alone. Reach out to family, friends, or professional caregivers for support.
Whether it’s a short break or long-term assistance, asking for help ensures both you and your loved one receive the care you need.
3—Explore New Skills
Learning new coping techniques, like mindfulness, stress management, or even a new hobby, can help you regain a sense of balance.
Skills such as meditation or relaxation techniques can make a significant difference in managing daily challenges.
4—Prioritize Self-Care
You can’t pour from an empty cup—make time for yourself.
Whether it’s a short walk, deep breathing exercises, or simply taking a moment to rest, self-care is not selfish. It’s essential.
Burnout doesn’t happen overnight, and recovery takes time.
But by implementing small changes and building a support system, you can continue providing care while maintaining your own well-being.
➡️ What’s one self-care habit that helps you manage stress as a care partner? Share in the comments!
#CarePartnerSupport #SelfCareForCaregivers #PreventBurnout #CaregivingTips #LightForPD #parkinsonsdisease #parkinsons #parkinsonsawareness #parkinsondisease #parkinsonsdiseaseawareness #parkinsonawareness #caregiver #goodnews
Parkinson’s disease is a progressive neurodegenerative disorder that affects movement, cognition, and overall quality of life—In its end stage, symptoms become more severe, requiring comprehensive care and support for both the individual and their caregivers.
Key Symptoms of End-Stage Parkinson’s:
➡️ Severe Motor Impairments
— Extreme rigidity and slowed movement (bradykinesia)
— Increased difficulty with balance and walking, leading to frequent falls
— Trouble initiating movement, requiring assistance with daily activities
➡️ Non-Motor Symptoms
— Autonomic dysfunction, including blood pressure fluctuations, urinary incontinence, and constipation
— Gastrointestinal issues such as decreased appetite and difficulty swallowing
— Sleep disturbances, including insomnia and excessive daytime drowsiness
➡️ Cognitive and Mental Health Challenges
— Cognitive decline, including memory loss, trouble with decision-making, and potential dementia
— Emotional symptoms such as increased anxiety, depression, and hallucinations
— Behavioral changes, including heightened emotional expressions and personality shifts
➡️ Providing Care in the End Stage
— Physical support—Assistance with hygiene, mobility, and medication management
— Emotional care—Providing comfort, companionship, and open communication
— Palliative and hospice care—Prioritizing quality of life, symptom management, and dignity
End-stage Parkinson’s is a difficult journey—but with the right support, comfort, and planning, individuals and their families can navigate this phase with compassion and care.
What has helped you or your loved one the most in managing late-stage Parkinson’s?
Share your thoughts below.
#ParkinsonsAwareness #EndStageParkinsons #Caregiving #PalliativeCare #QualityOfLife #LightForPD #parkinsonsdisease #parkinsons #parkinsonsawareness #parkinsondisease #parkinsonsdiseaseawareness #parkinsonawareness #caregiver #goodnews
Parkinson’s & Cognitive Changes: What You Should Know
Did you know that approximately 30% of people with Parkinson’s will experience some level of memory or thinking difficulties?
While Parkinson’s is widely known for affecting movement, cognitive changes—such as forgetfulness, trouble focusing, or difficulty making decisions—are also common but often less talked about.
These changes can range from mild cognitive impairment (occasional lapses in memory or slower thinking) to more significant challenges, impacting daily tasks and independence.
Though it can be concerning, there are ways to support brain health and maintain quality of life:
💡 How to Support Cognitive Function in Parkinson’s:
✔️ Stick to a structured routine – Familiar habits and schedules reduce confusion and make daily life easier.
✔️ Engage the brain – Activities like reading, puzzles, or learning a new skill help keep the mind active.
✔️ Stay physically active – Regular exercise has been shown to improve both cognitive and motor symptoms.
✔️ Prioritize sleep – Restorative sleep is crucial for memory and brain function.
✔️ Eat a brain-healthy diet – Foods rich in antioxidants, omega-3s, and healthy fats support cognitive health.
✔️ Communicate with healthcare providers – A neurologist or specialist can provide guidance on managing cognitive symptoms effectively.
If you or a loved one with Parkinson’s are experiencing memory lapses, difficulty concentrating, or slower processing, know that you’re not alone.
There are resources, strategies, and treatments that can help.
Have you noticed these changes in yourself or a loved one?
Let’s open up the conversation in the comments. ⬇️
#ParkinsonsAwareness #BrainHealth #CognitiveChanges #LivingWithParkinsons #LightForPD #parkinsonsdisease #parkinsons #parkinsonsawareness #parkinsondisease #parkinsonsdiseaseawareness #parkinsonawareness #caregiver #goodnews







Archived Content