Introduction
Welcome back to our exploration of groundbreaking advancements in Parkinson’s Disease (PD) treatment.
In Part 1, we dived into the potential of light therapy, other innovative non-pharmacological approaches like Focused Ultrasound and Transcranial Magnetic Stimulation, novel rehabilitation strategies, and the evolving landscape of drug candidates targeting core PD pathologies like alpha-synuclein, neuroinflammation, and specific genetic links.
Now, we turn our attention to even more revolutionary frontiers: biological game-changers like gene and stem cell therapies, and the incredible impact of technology in reshaping Parkinson’s care.
Biological Game-Changers: Gene and Stem Cell Therapies For Parkinson’s
-
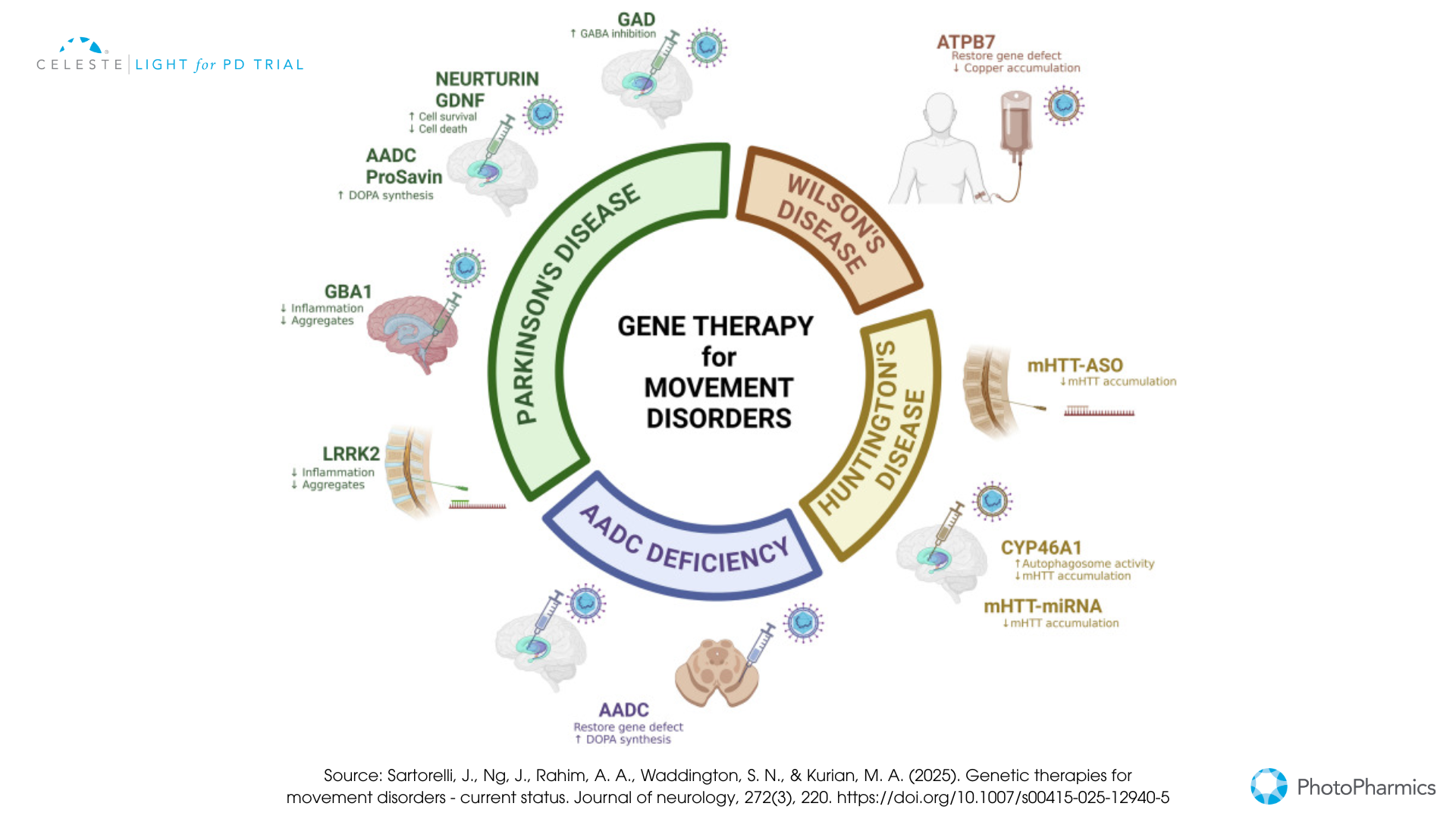
Gene Therapy
This approach involves delivering genetic material directly into specific brain cells to either restore a lost function, enhance the production of beneficial proteins, or correct a genetic defect.
- Dopamine Replacement Strategies: Some gene therapies aim to help the brain produce more dopamine by delivering genes like AADC (which converts levodopa to dopamine) or a combination of genes needed for dopamine synthesis (e.g., ProSavin, AXO-Lenti-PD).
- Neurotrophic Factors: Others focus on delivering genes that produce protective proteins like GDNF (Glial Cell Line-Derived Neurotrophic Factor), such as AskBio’s AB-1005 (currently in a Phase 2 trial), to shield dopamine neurons from further damage and potentially encourage recovery.
- Targeting Genetic Forms: For Parkinson’s linked to GBA1 mutations, gene therapies like PR001 (in Phase 1/2) aim to deliver a healthy copy of the GBA1 gene. These therapies typically use modified, harmless viruses (like AAV or lentivirus) as delivery vehicles and require neurosurgical procedures.
-
Stem Cell Therapy For Parkinson’s:
The goal here is bold: to replace the dopamine-producing neurons that have been lost.
- How it works: Scientists now take pluripotent stem cells—either human embryonic stem cells, hESCs, or induced pluripotent stem cells, iPSCs, derived from a patient’s own skin or blood. In the lab, they coax these cells to become young dopamine neurons. These new neurons are then surgically transplanted into the brain, usually the putamen.
- Current Status: Several teams worldwide are conducting early-phase clinical trials.
- BlueRock Therapeutics’ bemdaneprocel, derived from hESCs, has shown promising safety and initial efficacy signals in its Phase 1 trial, with some patients experiencing notable motor improvements and evidence of the transplanted cells surviving and functioning. A larger Phase 3 trial is anticipated.
- Aspen Neuroscience’s ANPD001 and a trial at McLean Hospital/Mass General Brigham are pioneering the use of autologous iPSCs (using the patient’s own cells), which avoids the need for immunosuppression. Early results from Aspen’s trial have also been positive regarding safety and initial motor benefits. While incredibly promising, stem cell therapy is still in its early days. Long-term efficacy, the ideal cell type and dosage, and managing potential side effects like graft-induced dyskinesia are key areas of ongoing research.
The progress in these pharmacological and biological frontiers is rapid, bringing the possibility of treatments that not only manage symptoms but also fundamentally alter the course of Parkinson’s disease closer than ever before.
Technology Leading the Way: DBS, Wearables, and AI in Parkinson’s
Technological advancements are profoundly reshaping how Parkinson’s disease is understood, managed, and treated, offering more personalized and precise interventions.
Innovations in Deep Brain Stimulation (DBS)
DBS has been a valuable treatment for advanced Parkinson’s for years, but it’s continuously improving.
- Adaptive DBS (aDBS) or Closed-Loop DBS: This is a major leap forward. Systems like Medtronic’s Percept™ PC with BrainSense™ technology, which recently received FDA approval, can sense and record brain signals (Local Field Potentials, or LFPs, particularly beta-band activity associated with Parkinson’s symptoms). The device then automatically adjusts the stimulation in real-time. This personalized approach aims to deliver optimal symptom control only when needed, potentially reducing side effects like speech issues or dyskinesia, and extending battery life. The ADAPT-PD trial supported its approval, demonstrating its safety and effectiveness.
- Enhanced Precision: Directional leads allow clinicians to “steer” the electrical current more precisely to the target area, avoiding stimulation of nearby structures that could cause side effects. Image-guided programming (IGP), using patient-specific MRI scans and models of the volume of tissue activated, helps clinicians optimize stimulation settings more efficiently, often leading to better outcomes, especially for those who had a suboptimal response to initial programming. Remote programming capabilities are also becoming more common, allowing for adjustments via telehealth and improving access to care.
Wearable Devices in Parkinson’s: Beyond the Clinic Walls
Wearable technology is empowering both patients and clinicians with continuous, real-world data.
- Symptom Monitoring: Small sensors (accelerometers, gyroscopes) worn on the body can track motor symptoms like tremor, bradykinesia (slowness), dyskinesia, and gait disturbances throughout the day. This objective data can help in fine-tuning medication timing and DBS settings, providing a clearer picture of how symptoms fluctuate.
- Drug Delivery: Innovation isn’t just in monitoring. In February 2025, the FDA approved “Onapgo,“ a wearable device that continuously delivers the drug apomorphine hydrochloride subcutaneously. This offers a new way to manage “off” episodes in advanced Parkinson’s, providing more consistent symptom control.
Artificial Intelligence (AI): The Smart Partner in Parkinson’s Care
AI is rapidly becoming an indispensable tool in nearly every aspect of Parkinson’s research and treatment.
- Earlier and More Accurate Diagnosis: AI algorithms are being trained to detect subtle, early signs of Parkinson’s that might be missed by human observation. This includes analyzing voice patterns (changes in pitch, clarity, or speed), subtle shifts in gait captured by sensors or even video, and even changes in handwriting or typing patterns. The goal is to identify the disease at its earliest stages when interventions might be most effective.
- Personalizing Treatment Strategies: As seen with aDBS, AI can interpret complex biological data to tailor treatments. It can also help predict which patients might respond best to certain medications or interventions and assist in optimizing drug dosages or rehabilitation plans.
- Accelerating Drug Discovery and Research: AI can sift through vast amounts of genetic, molecular, and clinical trial data to identify new potential drug targets, repurpose existing drugs for Parkinson’s, or design more efficient clinical trials. Initiatives like Grifols’ Chronos-PD are using AI to analyze plasma samples to find biomarkers that could predict Parkinson’s before symptoms even appear.
These technological tools are not just futuristic concepts; they are increasingly being integrated into clinical practice and research, heralding an era of more precise, data-driven, and patient-centered Parkinson’s care.
Navigating the Path Forward with Hope
The journey with Parkinson’s disease is undoubtedly challenging, but as we’ve explored, the horizon is bright with innovation. From the gentle promise of light therapy and the precision of focused ultrasound to the transformative potential of gene and stem cell therapies, and the smart assistance of AI and advanced DBS, researchers are leaving no stone unturned.
These advancements represent more than just scientific curiosity; they are fueled by a collective global effort to improve the lives of those affected by Parkinson’s and their families.
While many of these treatments are still in various stages of research and development, the pace of discovery is accelerating.
It’s crucial for patients and caregivers to stay informed and maintain an open dialogue with their healthcare team about new and emerging treatment options that might be relevant.
Participating in clinical trials, when appropriate, is also a vital way to contribute to this progress. The path to conquering Parkinson’s is a marathon, not a sprint, but with each new discovery and every dedicated researcher, clinician, patient, and caregiver, we move closer to a future where this disease no longer dictates the terms.
Hope is not just a sentiment; in the world of Parkinson’s research, it is an active, driving force.

Be Part of the Future of Parkinson’s Care with Celeste™
The future of non-invasive Parkinson’s therapy is here — and you could be part of it.
PhotoPharmics’ Light for PD™ trial is currently evaluating Celeste™, a breakthrough light therapy device designed to improve non-motor symptoms of Parkinson’s disease, such as poor sleep, fatigue, mood, and cognitive challenges.
What makes it different?
— It’s non-invasive and used from the comfort of home
— Designed specifically for people with Parkinson’s
— Part of a fully remote clinical trial, open to eligible participants across the U.S.
If you or a loved one is living with Parkinson’s and is interested in advancing treatment options that go beyond medication, we invite you to learn more about the Light for PD™ trial.
Visit lightforpd.com to check your eligibility and see how you can join the trial.
Help us move closer to better, more holistic care for Parkinson’s.






