Validating
Photo-Neuromodulation
Parkinson’s Phase 3 Pivotal Trial Underway
With basic science, animal work, and our early clinical work complete, our Celeste Light for PD phase 3 pivotal trial in Parkinson’s is well underway. Trial details include:
- Building on Our Phase 2 Trial. Celeste Light for PD closely follows the design and methodology of our phase 2 trial.
- Concurrence with FDA. Our clinical team met with FDA in pre-submission meetings to refine the protocol and statistical analysis to assure that if successful, the pivotal trial will provide the data necessary for market clearance.
- Successful Endpoints. The primary and key secondary endpoints agreed to by FDA for the phase3/pivotal trial, were statistically significant and clinically meaningful in the 92 patient, phase 2 trial.
- 350 Patients. Celeste Light for PD is fully enrolled with 350 patients. Half of the patients have received the Celeste device and half have received a comparator device.
- Greater than 90% Power. With 350 patients, the primary endpoint is powered at greater than 90%.
- Leading Clinical Trial Center. This is a remote clinical trial conducted by Ray Dorsey, MD, MBA and the team at the Center for Health and Technology (CHeT) at the University of Rochester. Dr. Dorsey is considered by many to be the world’s leading remote clinical trialist in movement disorders. CHeT has one of the best track records of conducting trials that lead to FDA approvals.

Read our recent announcement here. Additional details are available at www.clinicaltrials.gov (#NCT04453033). If you or someone you know would like to be considered for this trial, click here.
Trusted by Michael J. Fox Foundation
Fox Trial Finder
What Happens During/After the Trial? Featuring Jimmy Choi
Are you part of the Light for PD trial—or wanting to learn more about what we’re doing? This short video update answers key questions about what happens after the six-month study ends, including what to expect next and how continued access may work.
🎥 With special guest Jimmy Choi, Parkinson’s advocate and American Ninja Warrior, who shares his powerful story and why this research gives him hope.
Whether you’re involved in our trial or simply curious, this conversation will inform and inspire.
Early Clinical Trials
Parkinson’s Phase 2, 92 Patient Trial
Patients showed large, meaningful improvement in our phase 2, multi-national, randomized, double-blind, controlled trial. The trial enrolled 92 patients to assess the safety and efficacy of our photo-neuromodulation device (non-drug treatment) for motor and non-motor symptoms in Parkinson’s disease. Learn more at clinicaltrials.gov (#NCT02175472).
- Efficacy. We saw clinically meaningful improvements in combined both motor and non-motor function and quality of life. Patients enjoyed statistically significant improvements in tremor, sleep, fatigue, cognition, depression, anxiety, apathy and urinary function. Preliminary trial results can be viewed here.
- Safety. As anticipated, no device-related serious adverse events were reported in the trial. Patients experienced only benign and transitory side effects.
Supporting Research
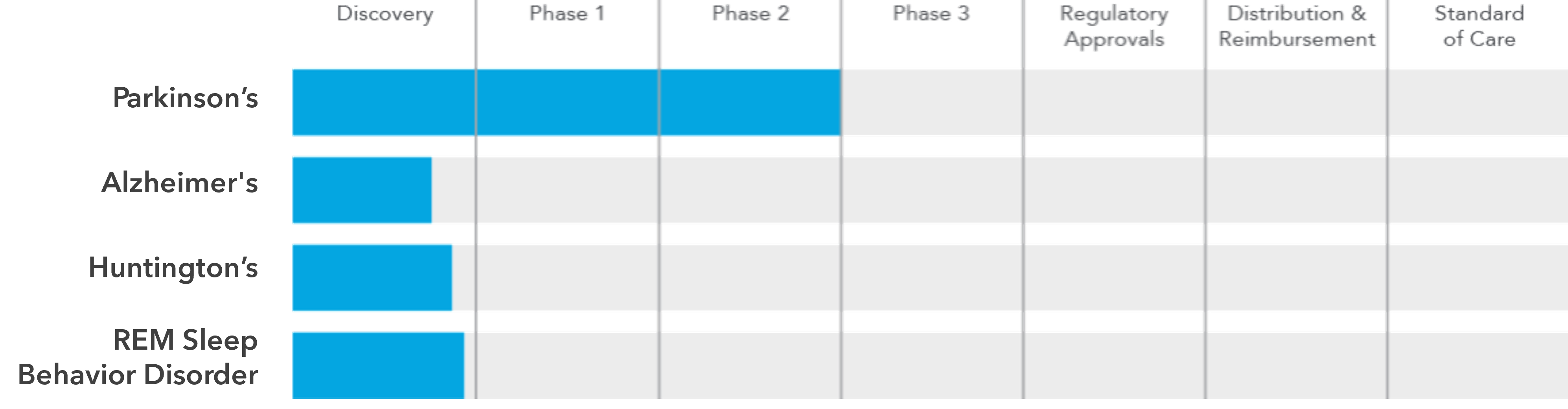
Pipeline
Other Neurodegenerative Diseases
Early data and our mechanistic work suggest that Celeste Photo-Neuromodulation may provide clinical benefit in other neurodegenerative and neuropsychiatric disorders. We are validating this data and will begin clinical studies to address these large unmet needs with the objective of providing novel, safe, and effective treatments to those who need it most.